

For our next look at this year’s Nobels, we thought we’d showcase the three brilliant researchers who share the prize for Chemistry. For those who need to come up to speed here’s Nature Briefing’s story, Chemistry Nobel for Supersponge MOFs
Chemists Susumu Kitagawa, Richard Robson and Omar Yaghi have won the Nobel Prize in Chemistry for developing the world’s most porous solid materials, known as metal-organic frameworks (MOFs). Structured like molecular scaffolding, MOFs contain vast caverns of internal space; Nobel committee chair Heiner Linke likens them to “Hermione’s handbag in Harry Potter — it can store huge amounts of gas in a tiny volume”. In the 30 years since they were first developed, they have become part of efforts to capture carbon from the air and remove ‘forever chemicals’ from water, among many other applications.Nature | 4 min read
Now, we in no way would distract from the accomplishments of Drs Kitaga or Yaghi. But what we want to do here is tell a very human story of how the third laureate, Dr Robson, got involved in the first place.
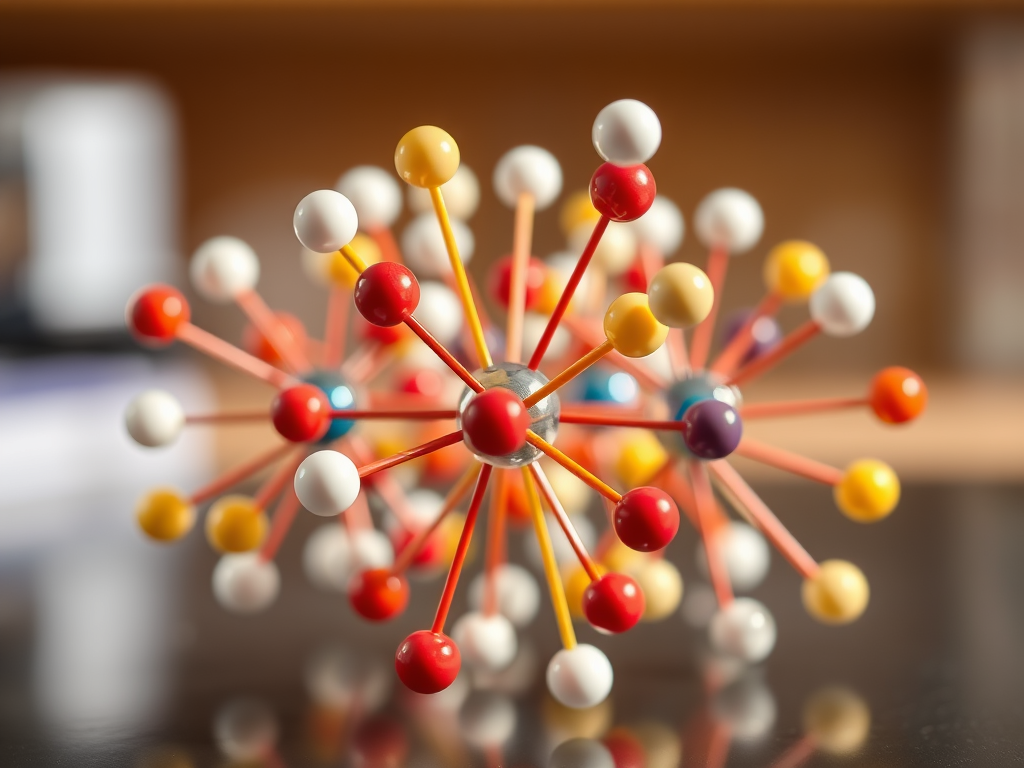
One day he was constructing large wooden models of crystal structures for undergraduate chemistry lectures at the University of Melbourne. These models—representing structures like sodium chloride and fluorite—were made from coloured wooden balls (atoms) connected by rods (bonds), carefully drilled at precise angles using trigonometric calculations. We’ve all seen them, they are stand by of every A level and undergraduate teaching room
As Robson assembled these models, he noticed something profound: the components seemed “invested with information,” naturally predisposed to form the intended structure. This observation led him to wonder: what if molecules could behave similarly—self-assembling into predictable, extended structures using chemical bonds instead of rods? That question planted the seed for MOFs, which he began exploring seriously about a decade later.
It’s funny how learning is a holistic thing. Research informs teaching. And teaching informs research. Oddly enough artists like Leonard Bernstein and Stephen Sondheim found the same thing, if you substitute “creative writing” for research. Perhaps its the idea of responding to questions, looking at the bigger picture. Or in Robson’s case, taking time out to play creatively with models. If you have found the same, oh readers, let us know. Meanwhile tell your Government to keep funding research and universities. As we saw in the last blog- they’ll get their money back.
#chemistry #nobel prizes 2025 #metal organic frameworks #carbon capture #climate change #science #research


